The following homogeneous liquid phase reactions are at equilibrium,
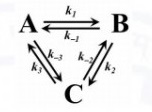
The values of rate constants are given by: $k_1 = 0.1 s^{-1}$, $k_{-1}=0.2 s^{-1}, k_2 = 1 s^{-1}, k_{-2}=10 s^{-1}, k_3=10s^{-1}$.
The value of rate constant $k_{-3}$ is _________ $s^{-1}$ (round off to $1$ decimal place)