Ethylene adsorbs on the vacant active sites $V$ of a transition metal catalyst according to the following mechanism.
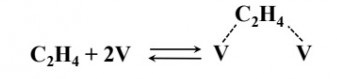
If $N_T, N_V$ and $N_{C_2H_4}$ denote the total number of active sites, number of vacant sites and number of adsorbed $C_2H_4$ molecules, respectively, the balance on the total number of active sites is given by
- $N_T=N_V+N_{C_2H_4}$
- $N_T=N_V+2N_{C_2H_4}$
- $N_T=2N_V+N_{C_2H_4}$
- $N_T=N_V+0.5N_{C_2H_4}$