Consider one mole of an ideal gas in a closed system. It undergoes a change in state from $L$ to $N$ through two different non-isothermal processes, as shown in the $P-V$ diagram (where $P$ is the pressure and $V$ is the molar volume of the gas). Process $I$ is carried out in a single step, namely $LN$ whereas process $II$ is carried out in two steps, namely $LM$ and $MN$. All the steps are reversible.
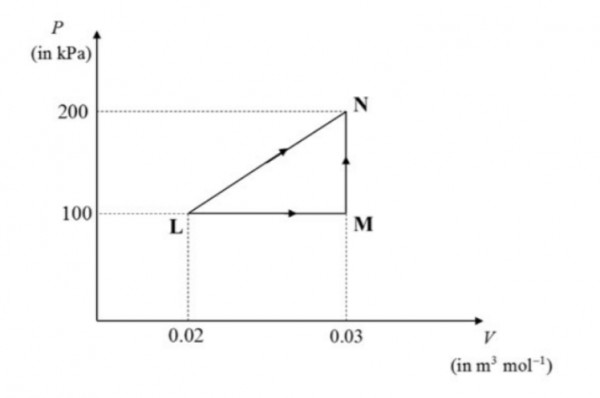
The net heat flowing into the system for process $I$ is $Q_1$ and that for process $II$ is $Q_{II}$. The value of $Q_I - Q_{II}$ (in $J$) is
- $250$
- $500$
- $1000$
- $1500$