It is decided to extract $A$ from a feed containing $20$ mol$\%$ $A$ and $80$ mol$\%$ $B$ in two ideal cross-current stages as shown below, using equal amount of pure solvent $C$ in each stage.
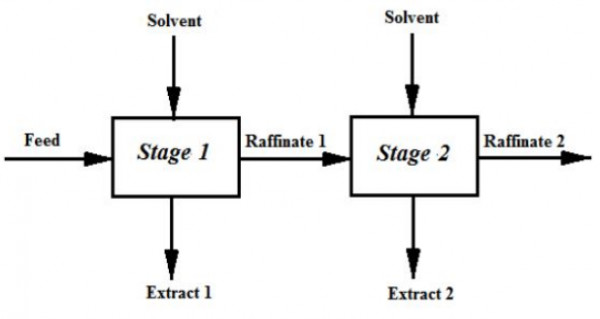
Components $B$ and $C$ are immiscible. $60\%$ of $A$ in the feed is extracted is $Stage\:1$. The equilibrium relation is given by $Y^{*}=1.5\:X$ where,
$X$=moles of $A$ per mole of $B$ in raffinate
$Y^{*}$=moles of $A$ per mole of $C$ in extract in equilibrium with raffinate
The mol $\%$ of $A$ in raffinate from $Stage\:2$ is ___________________ (rounded off to second decimal place).