In a laboratory batch setup, reaction of $P$ over a catalyst was studied at various temperature. The reactions occurring are
$$P\rightarrow 2Q\:;\:P\rightarrow R$$
At the end of one hour of operation, the batch contains $x_{P},\:x_{Q}$ and $x_{R}$ mole fraction of $P,Q$ and $R$ components, respectively. The mole fractions of product components ($x_{Q}$ and $x_{R}$) were found to vary linearly with temperature as given in the figure.
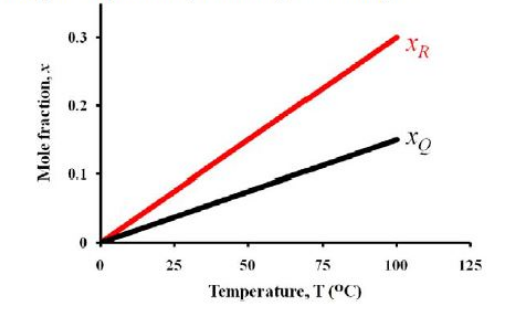
If the yield of $Q$ based on reactant $P$ consumed ($Y_{Q}$) at $25\:^{\circ}C$ was found to be $0.40$, then the value of $Y_{Q}$ at $60\:^{\circ}C$ is ________________ (rounded off to second decimal place).