The reaction A${_l}{_i}{_q}$ +B${_g}{_a}{_s}$ $\rightarrow$C${_l}{_i}{_q}$ +D${_g}{_a}{_s}$, is carried out in a rector followed by a separator as shown below
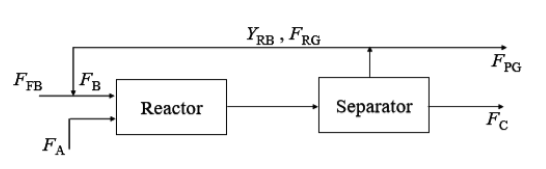
Notation:
Molar flow rate of fresh B is F${_F}{_B}$
Molar flow rate of A is F${_A}$
Molar flow rate of recycle gas is F${_R}{_G}$
Molar fraction of B in recycle gas is Y${_R}{_B}$
Molar flow rate of purge gas is F${_P}{_G}$
Molar flow rate of C is F${_C}$
Here, F${_F}{_B}$ =2 mol/s; F${_A}$ = 1 mol/s, F${_B}$/F${_A}$ = 5 and A is completely converted.
If Y${_R}{_B}$ = 0.3, the ratio of recycle gas to purge gas (F${_R}{_G}$/F${_P}{_G}$) is
- 2
- 5
- 7
- 10