Equilibrium data for a binary mixture of $E$ and $F$ at two different pressure is shown in the figure.
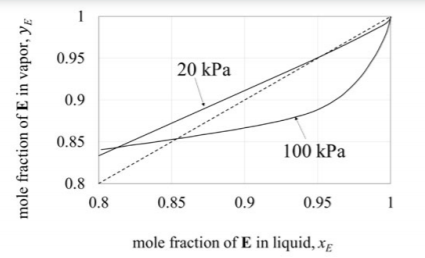
It is desired to process a feed containing $80 \text{ mol } \% E$ and $20 \text{ mol } \% F$, and obtain a product with a purity of $99.5 \text{ mol } \% E$. A sequence of two distillation columns, one operating at pressure $P_1$ and another at $P_2$, is employed for this operation, as shown below.
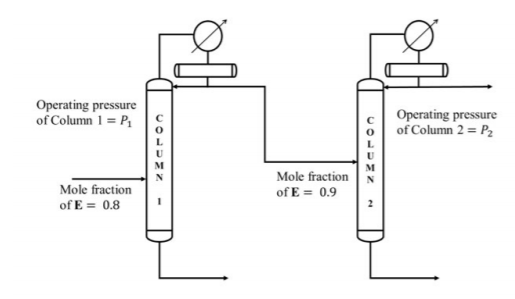
Mole fraction of $E$ in the distillate obtained from column $1$ is $0.9$. If the column pressures $P_1$ and $P_2$ are in $kPa$, which one of the following is correct?
- $P_1=100, P_2=20$, and high purity $E$ is recovered from the top of column $2$
- $P_1=100, P_2=20$, and high purity $E$ is recovered from the bottom of column $2$
- $P_1=20, P_2=100$, and high purity $E$ is recovered from the top of column $2$
- $P_1=20, P_2=100$, and high purity $E$ is recovered from the bottom of column $2$