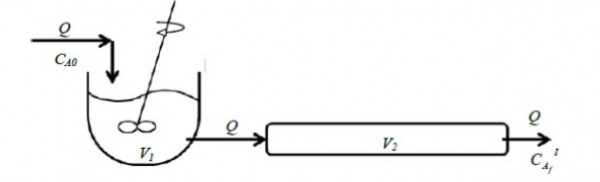
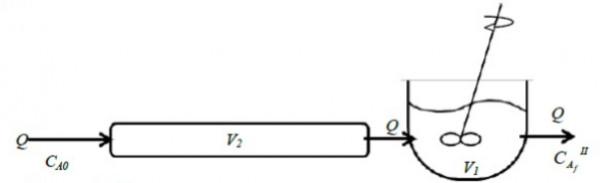
Consider two steady isothermal flow configurations shown schematically as Case $I$ and Case $II$ below. In Case $I$, a $CSTR$ of volume $V_{1}$ is followed by a $PFR$ of volume $V_{2}$, while in Case $II$ a $PFR$ of volume $V_{2}$ is followed by a $CSTR$ of volume $V_{1}$. In each case, a volumetric flow rate $Q$ of liquid reactant is flowing through the two units in series. An irreversible reaction $A{\rightarrow}$ products (order $n$) takes place in both cases, with a reactant concentration $C_{AO}$ being fed into the first unit.
Case $I$:
Case $II$:
Choose the correct option :
- $\frac{{C_{A}}_{f}^{I}}{{C_{A}}_{f}^{II}}>1\: for\: n=1$
- $\frac{{C_{A}}_{f}^{I}}{{C_{A}}_{f}^{II}}= 1\: for\: n=1$
- $\frac{{C_{A}}_{f}^{I}}{{C_{A}}_{f}^{II}}<1\: for\: n=1$
- $\frac{{C_{A}}_{f}^{I}}{{C_{A}}_{f}^{II}}= 1\: for\: n>0$