The volumetric properties of two gases $M$ and $N$ are described by the generalized compressibility chart which expresses the compressibility factor $(Z)$ as a function of reduced pressure and reduced temperature only. The operating pressure $(P)$ and temperature $(T)$ of two gases $M$ and $N$ along with their critical properties $(P_{C},T_{C})$ are given in the table below.
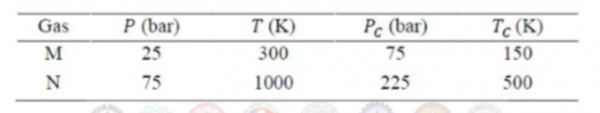
$Z_{M}$ and $Z_{N}$ are the compressibility factor of the gases $M$ and $N$ under the given operating conditions, respectively.
The relation between $Z_{M}$ and $Z_{N}$ is
- $Z_{M}=8Z_{N}$
- $Z_{M}=3Z_{N}$
- $Z_{M}=Z_{N}$
- $Z_{M}=0.333Z_{N}$