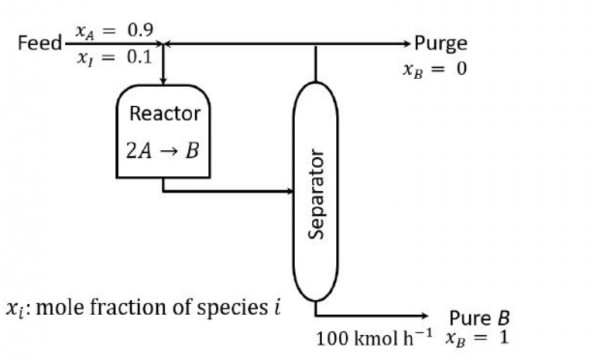
Consider the process in the figure for manufacturing $B$. The feed to the process is $90 \mathrm{~mol} \% A$ and a close-boiling inert component $I$. At a particular steady-state:
- $B$ product rate is $100 \mathrm{kmol} \mathrm{h}^{-1}$
- Single-pass conversion of $A$ in the reactor is $50 \%$
- Recycle-to-purge stream flow ratio is $10$
The flow rate of $A$ in the purge stream in $\mathrm{kmol} \mathrm{h}^{-1}$, rounded off to $1$ decimal place, is $\_\_\_\_\_\_\_\_$