The elementary, irreversible liquid-phase, parallel reactions, $2A\rightarrow D$ and $2A\rightarrow U$, take place in an isothermal non-ideal reactor. The $C$-curve measured in a tracer experiment is shown in the figure, where $C(t)$ is the concentration of the tracer in $g/m^{3}$ at the reactor exit at time $t$ (in min).
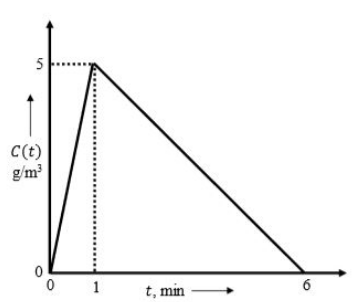
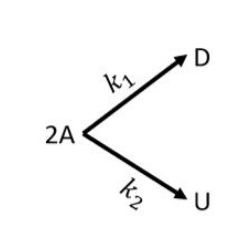
The rate constants are $k_{1}=0.2$ Liter/(mol min) and $k_{2}=0.3$ Liter/(mol min). Pure $A$ is fed to the reactor at a concentration of $2$ mol/Liter. Using the segregated model, the percentage conversion in the reactor is ___________________ (rounded off to the nearest integer)