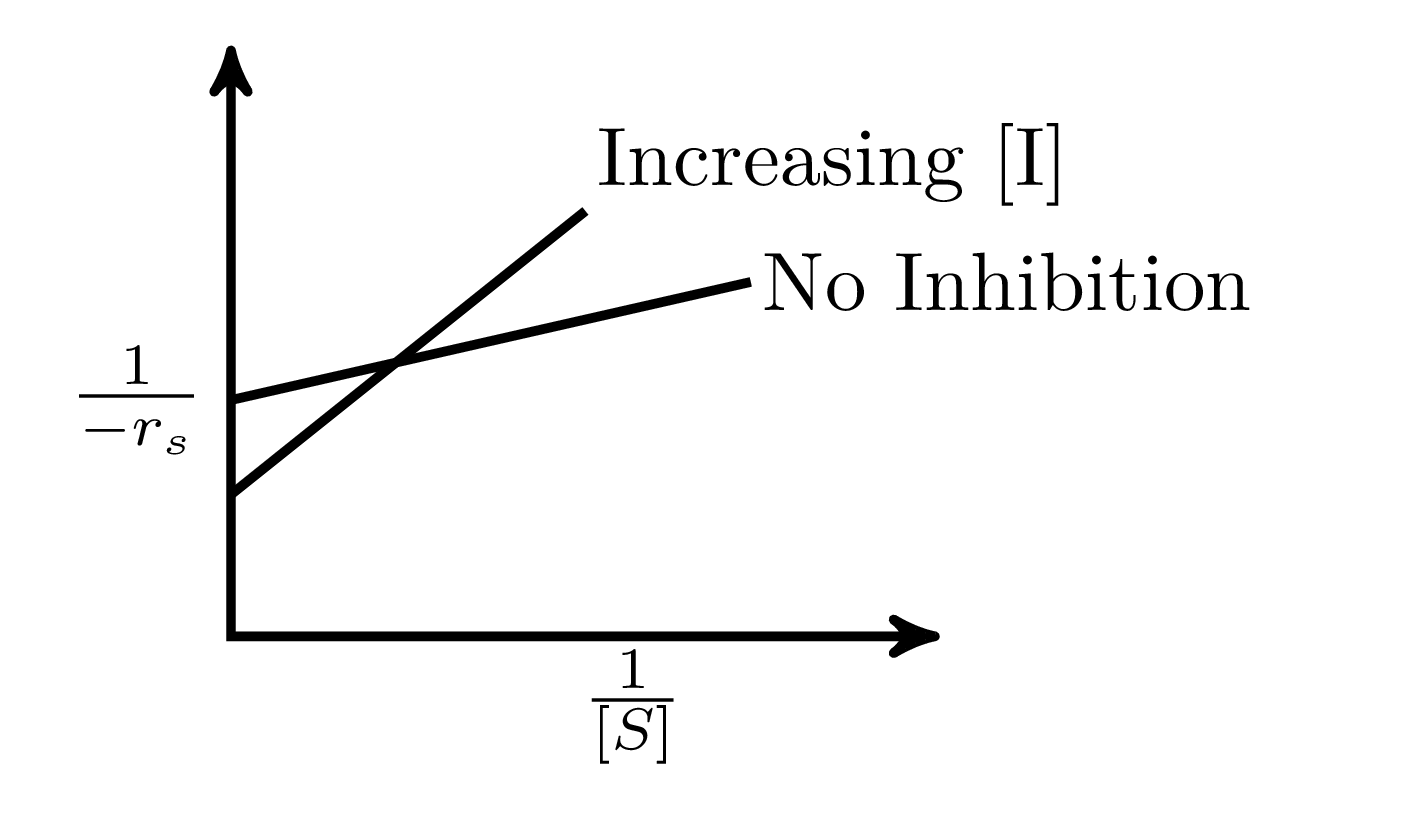
In an enzymatic reaction, an inhibitor $(I)$ competes with the substrate $(S)$ to bind with the enzyme $(E)$, thereby reducing the rate of product $(P)$ formation. The competitive inhibition follows the reaction mechanism shown below. Let $[S]$ and $[I]$ be the concentration of $S$ and $I$, respectively, and $r_{s}$, be the rate of consumption of $S$. Assuming pseudo-steady state, the correct plot of $\frac{1}{-r_{S}}$ vs $\frac{1}{[S]}$ is
$$E + S _{\xleftarrow[K_{2}]{}}^{\xrightarrow[]{K_{1}}} E \cdot S \xrightarrow[]{K_{3}} E + P$$ $$E + I _{\xleftarrow[K_{-I}]{}}^{\xrightarrow[]{K_{I}}} E \cdot I$$
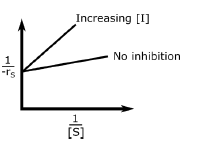
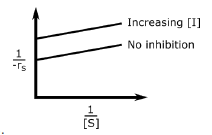
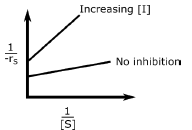
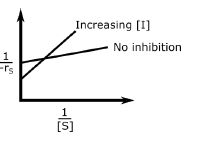
A.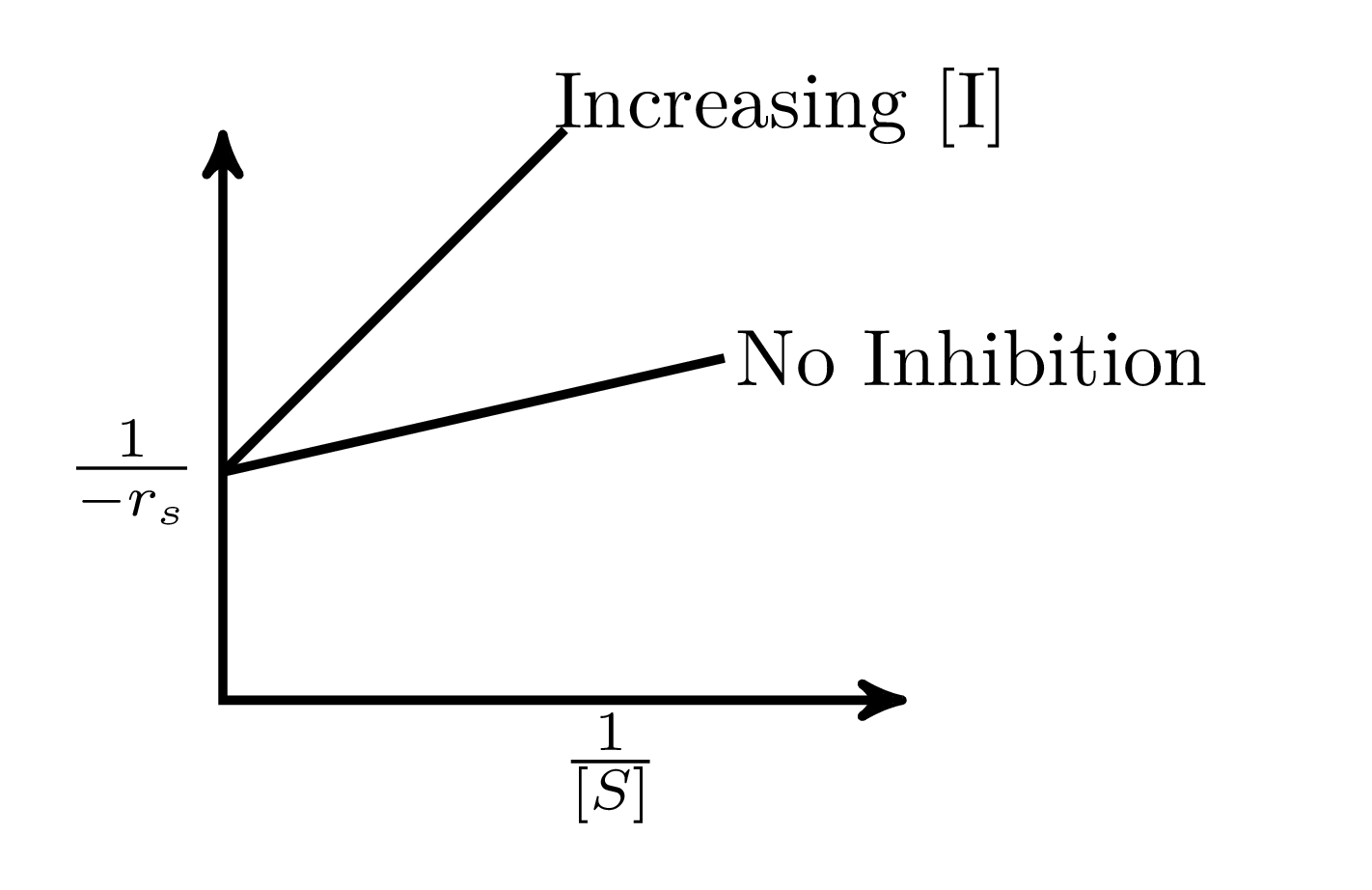
B. 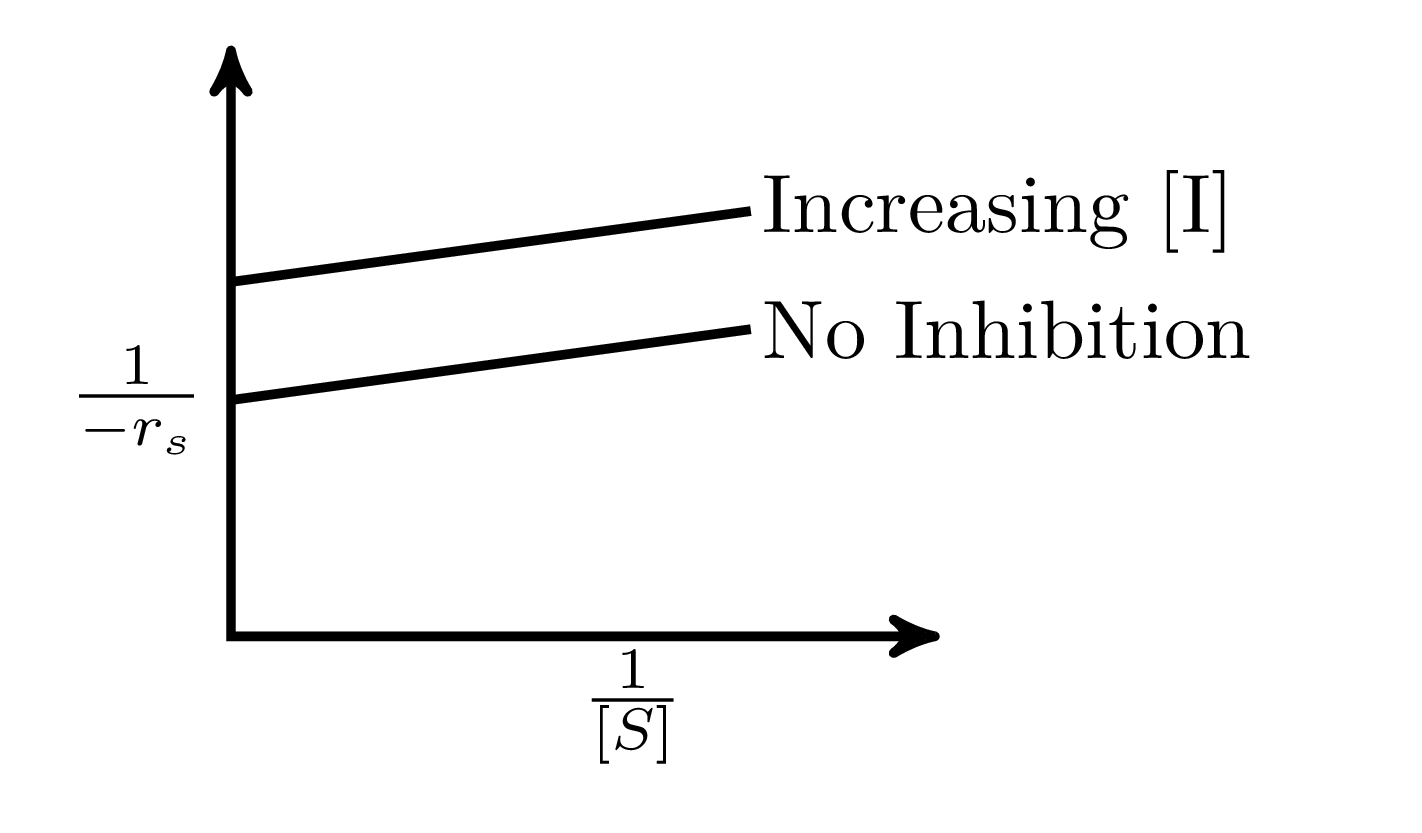
C. 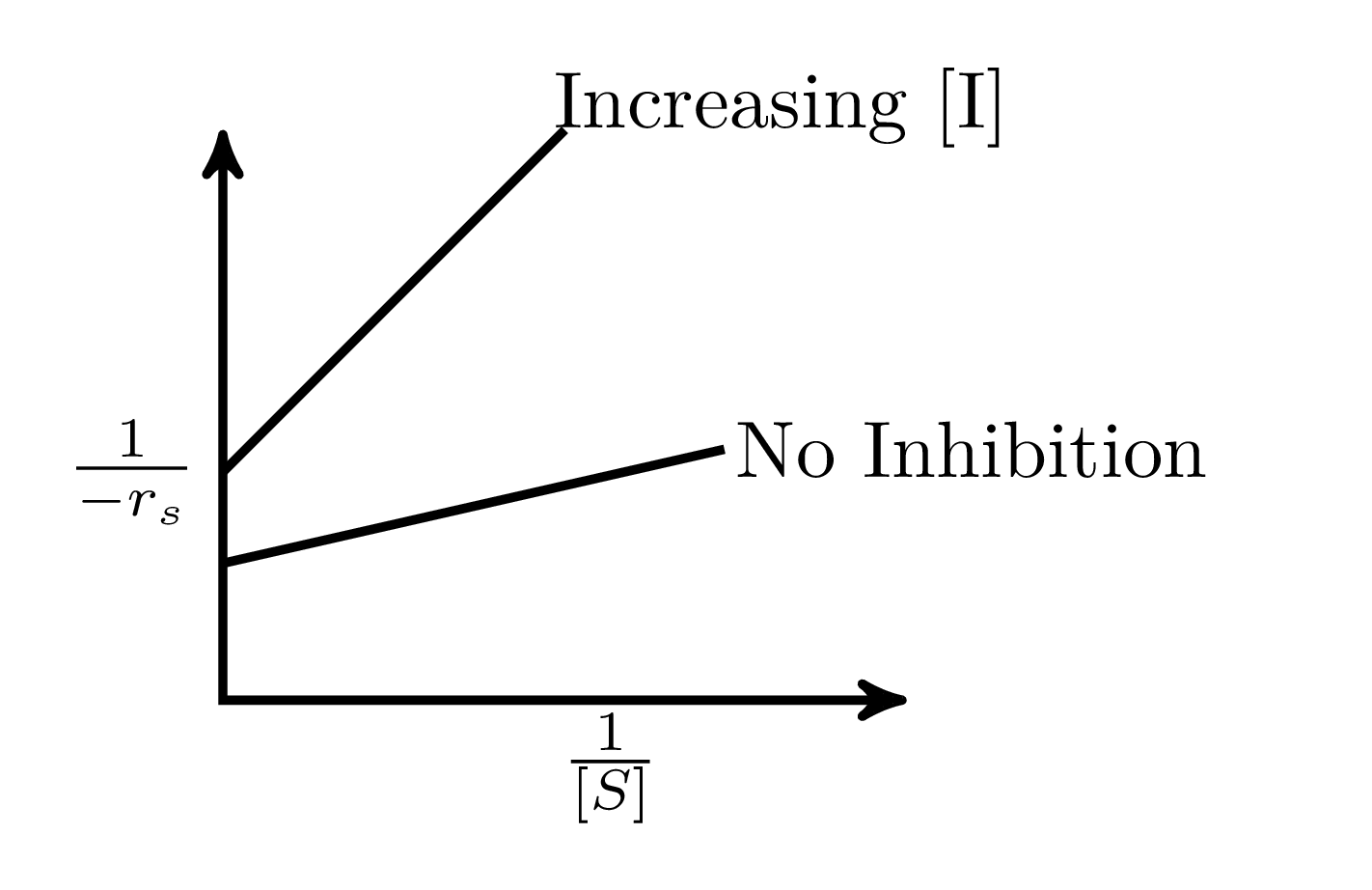
D.