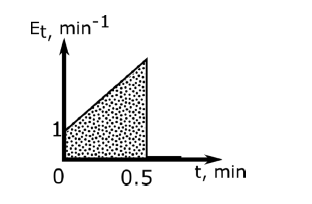
An elementary irreversible liquid-phase reaction, $2P \overset{k}{\rightarrow}Q$, where the rate constant $k = 2\:L\:\text{mol}^{-1}\:\text{min} ^{-1}$, takes place in an isothermal non-ideal reactor. The $E$-curve in a tracer experiment is shown in the figure. Pure $P \left(2\:\text{mol}\:L^{-1}\right)$ is fed to the reactor. Using the segregated model, the percentage conversion of $P$ at the exit of the reactor is _____________ $\%$ (round off to the nearest integer)